Part 2
XLP
"In this case what the claimants are really complaining about in terms of safety is not that a large head MoM articulation can cause ARMD, but that because of the incidence of ARMD that they say materialised or can be reliably predicted, Pinnacle Ultamet prostheses had a materially higher risk of early failure than comparator non- MoM prostheses."
Note: ARMD = Adverse Reaction to Metal Debris i.e. tissue damage near the hip joint, metal ions in blood, pseudo tumours.
My complaint, like most others in the Leigh Day group, was EXACTLY that DePuy's Pinnacle Ultamet hip could, and DID, damage me (see hospital notes below) ...

Part of my hospital notes - October 2014
... at the time, I urgently wanted to have this toxic MOM hip removed from my body.
In the early days of Pinnacle Ultamet no one knew how poor they would be in terms of durability and safety - not even DePuy. Patients expectations were that they would be better than other prostheses, because of DePuy's claims.
-0-
At the trial, the DuPuy argued (as they had in U.S. Trials) that the only reasonable way to assess Ultamet performance was to compare it with other hips on the market in 2002, it was introduced.
A few points ...
1. If DePuy had performed effective testing, before releasing Ultamet to be tested on the public, they would have know how reliable and durable it would or would not been. As far as I can see, no successful 'accelerated life testing' was performed. If it had been, then DePuy would have broadcast the results.
2. If DePuy were largely depending on testing their hip on patients from 2002, then there'd be no valid answers available until at least 2004, when just over 1000 Pinnacle (not necessarily Ultamet) hips had been fitted. See below.
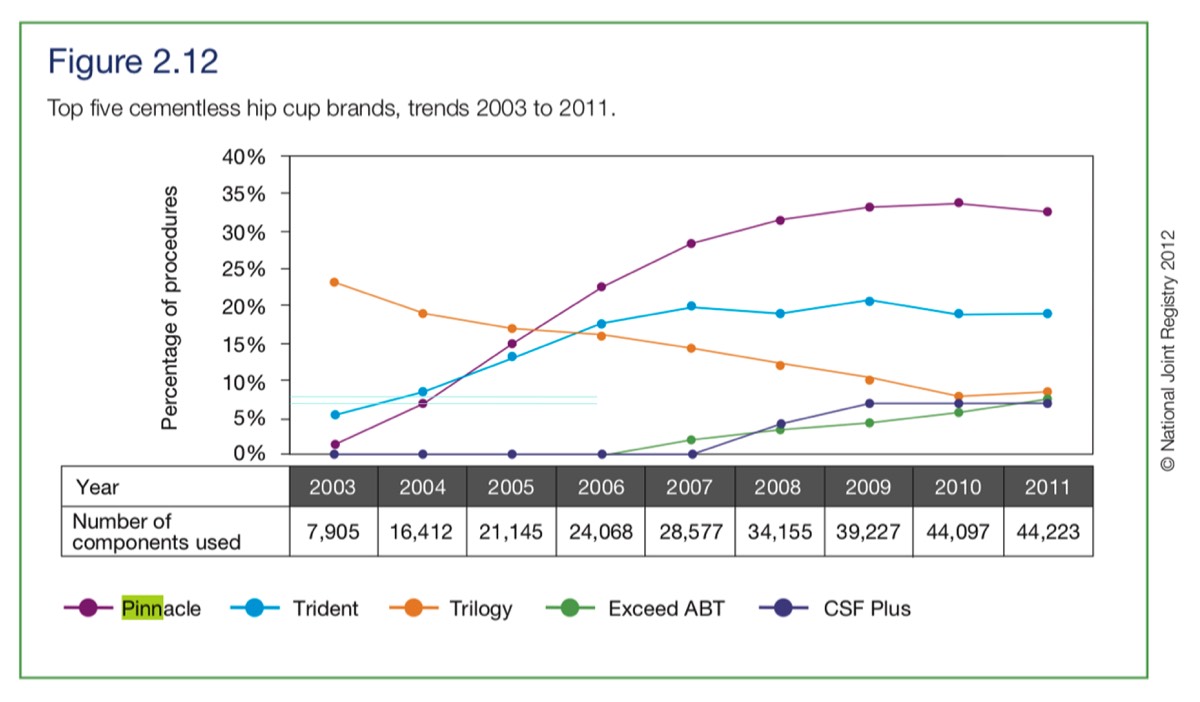
3. The NJR graph above indicates that in 2003, approximately 99 Pinnacle hips were used (not all Ultamet). So in 2002 there must have been less than 50 fitted. If a patient had a THA in 2004, why would they compare performance with 2002 hip? It's my view that patients trusted their surgeon's advice that it should last 15 or more years.
4. It seems to me that DePuy wanted the court to agree to comparison with older prostheses, so that their Pinnacle Ultamet might look less bad. A bit like comparing a car with a cart.
As you will see in Part 4 of this series of blogs, the only hip which can make Ultamet look less bad is DePuy's own ASR hip which they recalled after 5 years in August 2010. (Early in 2010, DePuy said they were phasing out the ASR Hip because of declining sales. Does that sound familiar?
-0-
The 311 Leigh Day Claimants wanted to use the UK National Joint Register's data to provide a way of comparing the Pinnacle MOM hip's performance with other hips being used at the time. DePuy argued that the advent of a new form of polyethylene hip bearing would make a performance comparison invalid.
In Paragraph 313 of her verdict, Mrs Justice Andrews wrote:
"If data relating to MoP hip prostheses includes both metal on conventional polyethylene and metal on cross-linked polyethylene or HXLPE articulations, as it undoubtedly does in the NJR database and in the SHAR 2014 report, it is likely to be less reliable for comparison purposes than if it relates to metal on conventional polyethylene alone. The whole purpose of the intentional cross-linking was to produce a harder wearing and more durable surface; if and to the extent that succeeded (as the short-term scientific information on its performance suggests that it has) one would expect data relating to the cross-linked product or products to improve the CRR even in the short-term. There was some debate at trial about whether the inclusion of HXLPE in the NJR data made a statistically appreciable difference to the CRR for MoP over 10 years. Bearing in mind all the non-statistical evidence, as well as the history leading to the development of the alternative harder bearing surfaces, one would expect the data to demonstrate metal on HXLPE articulations to have an appreciably better CRR than metal on conventional polyethylene."
Note: SHAR = Swedish Hip Arthroplasty Register, and CRR = The rate of hip prosthesis revision operations.

Well, XLPE was first used in the 1990s - four years before Ultamet appeared in the UK.
Even DePuy had produced their Marathon XLPE in 1997 (click here and here).
There was therefore no reason to exclude Metal-on-XLPE from performance comparisons, and absolutely no reason to use SHAR data.
In Paragraph 322 of her verdict, Mrs Justice Andrews wrote:
"Whatever its limitations, SHAR was the registry which informed the orthopaedic community’s understanding of survivorship of hip implants at the relevant time. Both generic orthopaedic experts confirmed its importance as a source. Mr Whitwell said in his evidence that “we still quote from it quite reliably because it’s the longest registry”. I find that the SHAR registry is the most appropriate source of relevant and reliable information about the actual and likely performance of conventional MoP prostheses over the short and longer term. The NJR, which was only set up in 2003, has no data pertaining to any 10-year period up to and including 2002, when the Ultamet came onto the market, and its data relating to the performance of MoP prostheses over later periods is confounded to an unknown and unascertainable extent by the inclusion of data pertaining to HXLPE and products that came onto the market later than the Pinnacle Ultamet, even before one even begins to consider other confounding factors. If the SHAR registry is an unreliable source of information about the 10-year survivorship or CRR of MoP prostheses for the purposes of the comparative exercise, the NJR is even less reliable."
Confounding factors are things which can make statistical analysis tricky. In this context the factors might include: age, BMI, activity, gender, prosthesis technology.
As mentioned above ... XLPE was first used in 1998 - four years before Ultamet appeared in the UK. Even DePuy had their Marathon XLPE in 1997 (click here and here). So a product introduced in 2002 should compare favourably with one introduced in 1998. There was, as I've said, absolutely no reason to exclude Metal-on-XLPE from performance comparisons, and therefore no reason to use SHAR data.
The important result of all this will be included in the next part of this series.